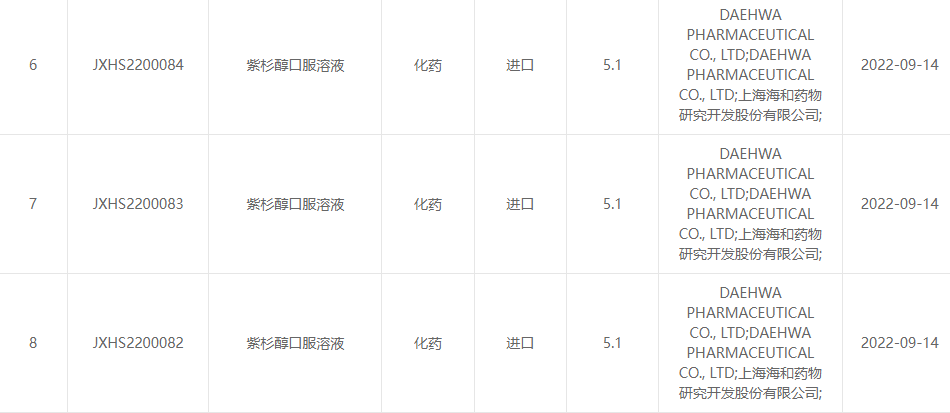
NgoSepthemba 13, 2022, i-Shanghai Haihe Pharmaceutical Research and Development Co., Ltd. kanye ne-Daehwa Pharmaceutical Co., Ltd. bamemezele ngokuhlanganyela ukuthi i-paclitaxel oral solution (RMX3001) eyakhiwe ngokuhlanganyela yilawa maqembu womabili igunyazwe ngokusemthethweni yiCentre for Drug. Ukuhlolwa (CDE) Kokuphathwa Kwezidakamizwa Zombuso.(Inombolo yokwamukela: JXHS2200082 izwe, JXHS2200083 izwe, JXHS2200084 izwe).
Umthombo wesithombe: I-State Drug Administration
I-Paclitaxelisetshenziswa kabanzi ekwelapheni izimila ezibulalayo ezahlukahlukene njengomdlavuza wamaphaphu, umdlavuza webele, umdlavuza wesibeletho, umdlavuza wekhanda nentamo, nomdlavuza wesisu.I-polymerization yamaprotheni, ukuhlanganiswa kwe-microtubule, ukuvimbela ukuchithwa kwe-depolymerization, ngaleyo ndlela kuzinzisa ama-microtubules futhi kuvimbele i-mitosis yamangqamuzana omdlavuza futhi kubangele i-apoptosis, ngaleyo ndlela kuvimbele ngokuphumelelayo ukwanda kwamangqamuzana omdlavuza nokudlala umphumela wokulwa nomdlavuza.
Njengamanje, izingxenye eziningi zomhlaba zisebenzisa i-paclitaxel ewumjovo, odinga ukwakhiwa futhi ulawulwe nge-drip efakwa emthanjeni esibhedlela.Iziguli kudingeka zibuyele esibhedlela njalo, futhi kuzoba nokusabela okungekuhle endaweni yomjovo.Ngakho-ke, ukuthuthukiswa kwamalungiselelo e-oral paclitaxel bekulokhu kuyindawo eshisayo ocwaningweni lwemboni..
I-RMX3001 iwukwenziwa ngomlomo kwe-paclitaxel eyakhiwe yi-Dahua Pharmaceutical ngokusekelwe kubuchwepheshe bayo obusha be-lipid obuzikhandlayo bokulethwa kwezidakamizwa.Kugunyazwe i-Korean Food and Drug Administration ngo-September 2016 (igama lokuhweba i-Liporaxel), futhi inkomba iwukwelashwa okuthuthukisiwe noma komugqa wesibili womdlavuza wesisu we-metastatic noma umdlavuza wesisu ovela endaweni.Ngokombiko wabezindaba ovela e-Haihe Pharmaceuticals, i-Liporaxel ingumkhiqizo wokuqala we-oral paclitaxel othuthukiswe ngempumelelo futhi wavunyelwa ukumakethwa emhlabeni kuze kube manje.NgoSepthemba 2017, i-Haihe Pharmaceutical yathola i-R&D, amalungelo okukhiqiza nawokuthengisa omkhiqizo ezweni lase-China, e-Hong Kong, e-Taiwan nase-Thailand e-Dahua Pharmaceuticals.
Ukufakwa kuhlu kwe-RMX3001 e-China kusekelwe ikakhulukazi kwilebula engahleliwe, evulekile, elawulwa ngokuhambisanayo, umklamo ongekho ngaphansi, ukuhlolwa komtholampilo kweSigaba sesi-3 esiphakathi nendawo, okuhloswe ukuqhathanisa ukwelashwa komugqa wesibili we-paclitaxel oral solution RMX3001 kanye I-paclitaxel injection (Taxol) Ukusebenza kahle nokuphepha ezigulini ezinomdlavuza wesisu othuthukile.Ucwaningo lwenziwe ngokuhlanganyela nguSolwazi Li Jin wase-Shanghai Oriental Hospital kanye noSolwazi Qin Shukui wase-Nanjing Jinling Hospital njengabaphenyi abakhulu.
UDkt. Ruiping Dong, oyiChief Executive Officer ye-Haihe Pharmaceuticals, uthe: “Ukwamukelwa kwesicelo se-paclitaxel oral solution (RMX3001) kungesinye igxathu elibalulekile le-Haihe Pharmaceuticals, futhi ngibonga kakhulu kubaphenyi bemitholampilo kanye neziguli ezibambe iqhaza ohlelweni lwethu. icala.Umdlavuza wesisu osethuthukile Kusenesidingo esikhulu sokwelashwa esingakafinyelelwa, futhi sithemba ukuletha izindlela zokwelapha ezisezingeni eliphezulu nezilula kakhulu ezigulini zaseChina nasemhlabeni wonke ngokushesha ngangokunokwenzeka.”
I-Yunnan Hande Biotechnology Co., Ltd. ibigxile ekukhiqizweni kwe-paclitaxel iminyaka engama-28.Ingumkhiqizi wokuqala ozimele emhlabeni womuthi we-anticancer osuselwa esitshalweni ugunyazwe yi-US FDA, European EDQM, Australian TGA, China CFDA, India, Japan kanye nezinye izikhungo zokulawula zikazwelonke.ibhizinisi.Uma ufuna ukuthengaI-Paclitaxel API,sicela usithinte ku-inthanethi.
Isikhathi sokuthumela: Sep-14-2022